Protect Water ResourcesUnderstand Pesticide Movement
GWQ-00547 View this publication in PDF form to print or download.
The impact of pesticides on surface and groundwater has become an increasingly debated topic. For years the use of pesticides has helped to minimize the damage done by pest populations to agricultural crops while maximizing production. However, the same characteristics that make pesticides effective for pest control can create a potential for groundwater contamination.
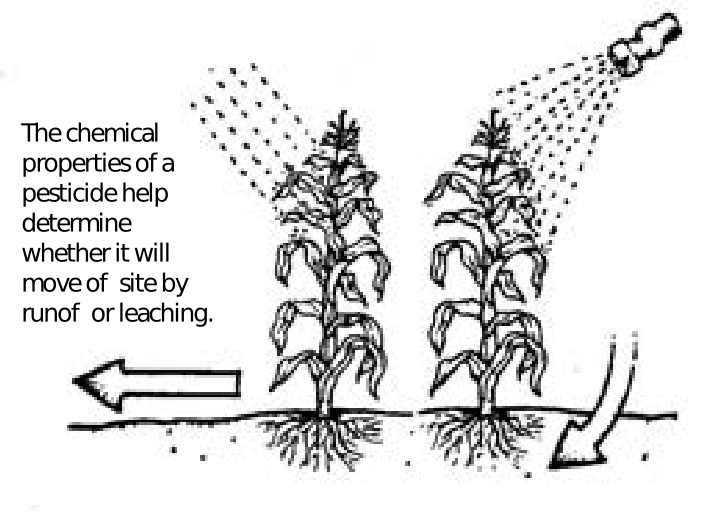
The information presented here is designed to help pesticide users better understand how chemical properties, soil characteristics, site conditions and management practices are likely to affect pesticide movement.
Who’s Responsible for Minimizing Pesticide Damage?
The user is ultimately responsible for minimizing the potential for pesticide movement into nontarget areas. The primary consideration should be whether the chemical is actually needed or if other nonchemical practices could be used. If pesticides are needed, it is essential that the user understand the factors that control the movement and persistence of pesticides in the environment.
Chemical Properties
Aqueous solubility refers to the capacity of a pesticide to dissolve in water. This capacity, commonly expressed as parts per million (ppm), i.e., one part of pesticide in one million parts of water, helps to define a pesticide’s leaching potential. Generally, the higher the solubility, the greater chance that the pesticide may leach to groundwater. Pesticides with a solubility of less than 1 ppm tend to remain on the soil surface and are normally attached to soil particles; they tend not to be leached, but may move with soil sediment into surface waters via soil erosion. Pesticides with a solubility greater than 30 ppm are more likely to leach or move downward through the soil profile if they are not absorbed by plants, degraded or bound to the soil.
Read pesticide labels carefully
for precautionary information. Additional details can be found on the product Safety Data Sheet. For further information or product recommendations please contact your local Cooperative Extension Service office.
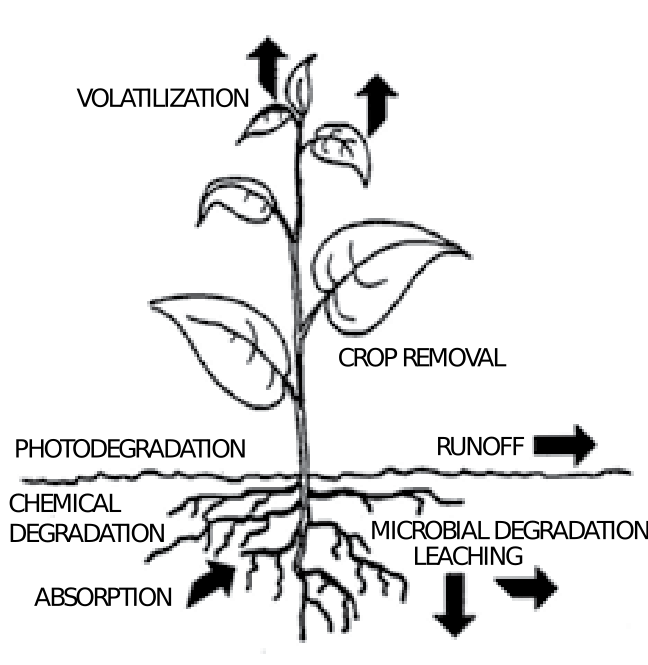
Persistence is a measure of how long it takes a pesticide to degrade, often expressed as half-life, the time required for a pesticide to degrade to one-half of the original amount applied. Most degradation occurs at the soil surface or in the root zone, and is mainly due to the activity of soil bacteria or fungi and breakdown by sunlight. Degradation is usually slower in cool soils. In general, the longer the half-life, the greater the potential for groundwater contamination. Scientists have classified pesticides with a half-life of less than 30 days as nonpersistent, whereas those with a half-life greater than 100 days are classified as persistent.
Adsorption is a process which results in the chemical bonding of a pesticide to soil particles. It is expressed as the “sorption index,” abbreviated Koc. The more strongly a chemical is adsorbed, the higher the Koc value will be. A high Koc, greater than 1000, indicates that a pesticide is very strongly attached to the soil and that it is not likely to move unless soil erosion occurs. Those pesticides with values less than 300 tend to move more easily with water and have a greater potential for leaching or movement with surface water. Typically, highly soluble pesticides are more weakly adsorbed in a given soil than those with low solubility. Adsorption may be the single most important characteristic for determining environmental fate. The sorption index of some pesticides is variable and can be greatly influenced by factors such as soil pH and soil organic matter.
Vapor pressure is a measure of volatility. Volatile pesticides (those with high vapor pressure) easily change form, from a solid or liquid to a gas. The gas is usually not a direct threat to water resources, but drift may cause harm in nontarget areas or contribute to pesticide levels in rain. Excessively volatile pesticides are lost quickly from warm, moist soil surfaces. Application of volatile pesticides is best done when there is little air movement and when soils are dry and cool. In high-humidity areas, volatility is unlikely.
Soil Characteristics
Soil permeability is a measure of how quickly water tends to move downward through the soil. It is primarily determined by the texture and structure of a soil. Clay or loam soils are generally less permeable than those composed of larger soil particles such as sand or gravel.
Organic matter. The presence of organic matter or clay increases the soil’s ability to adsorb or tie up pesticides. Increasing soil adsorption can reduce pesticide movement downward, but it may also result in the need for a higher application rate of soil-applied pesticides due to diminished pesticide activity. Detailed application information is provided on the product label.
Properties, characteristics and conditions that can affect pesticide movement
Risk of Groundwater Contamination
| Chemical Properties | Low Risk | High Risk |
|---|---|---|
| Water solubility | low | high |
| Soil adsorption | high | low |
| Persistence | low | high |
| Soil Characteristics | ||
| Texture | fine clay | coarse sand |
| Organic matter | high | low |
| Site Conditions | ||
| Pore spaces | few, small | many, large |
| Depth to groundwater | deep, 100 ft. or more | shallow, 20 ft. or less |
| Rainfall or irrigation | small volumes at infrequent intervals | large volumes at frequent |
| intervals |
Based on McBride, D.K. 1989. North Dakota State University Extension Service
Phil Kaspari, Agriculture and Horticulture Agent. Originally prepared by Wayne Vandre, former Extension faculty, Agriculture and Horticulture, and Mary Comeau, former IPM Coordinator.
Reviewed November 2021
